Every year, world pharmacopoeias convene through the International Meeting of World Pharmacopoeias (IMWP) to share experience and expertise and find ways of working together to synchronize their efforts to improve public health outcomes. Although different pharmacopoeias cover different countries or regions, they all work to protect public health by creating and making available public standards to help ensure the quality of medicines.
The fourteenth IMWP, which was hosted by the Pharmacopoeia of the United Mexican States (FEUM), was held in Mexico City, Mexico, from 8–9 November 2023 (with a stakeholder event on the 10 November 2023). In total, 53 participants, representing 15 national and 1 regional pharmacopoeias, attended the meeting. Natalia VOLOVYK, Deputy Director for Quality at the Ukrainian Scientific Pharmacopoeial Center for Quality of Medicines, represented the State Pharmacopoeia of Ukraine (SPhU).
During the meeting, world pharmacopoeias shared their experience and information on news and work undertaken over the past years to support national and global public health.
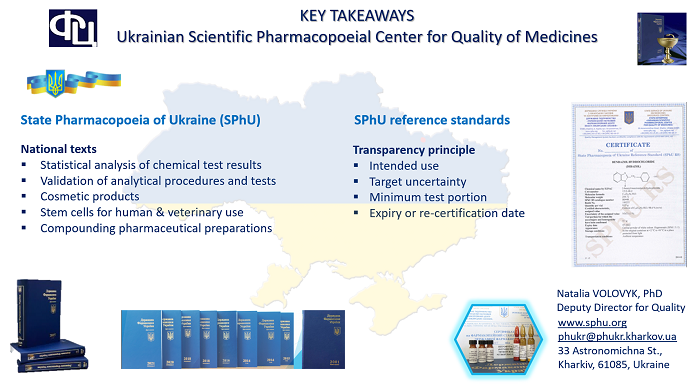
Dr Volovyk provided a comprehensive overview of the history, mission, and main activities of the Ukrainian Scientific Pharmacopoeial Center for Quality of Medicines. She underlined that despite the significant challenges posed by Russia’s ongoing war against Ukraine, work has continued in all key areas, demonstrating the resilience of the centre’s staff. Speaking about centre’s achievements, Natalia VOLOVYK presented key national texts of the State Pharmacopoeia of Ukraine, including Statistical analysis of chemical test results, Validation of analytical procedures and tests and Mesenchymal stem cells for human and veterinary use, and reported about metrological approaches to certification of pharmacopoeial reference standards using the principle of transparency.
The Pharmacopoeial Discussion Group provided an update on its activities, including its expansion to include the Indian Pharmacopoeia Commission as a fourth member.
IMWP members reviewed progress of ongoing joint projects on COVID-19-related activities and internal collaboration and communication methods and identified new areas for collaboration around defining what IMWP does, and how and environmental sustainability.
Participants of the fourteenth IMWP agreed to continue working together to exchange information and knowledge that can help assure medicines quality.