On 10th October 2023, colleagues from the Ukrainian Pharmacopoeial Center participated in the webinar titled "Secondary Standards – Considerations in Traceability to Pharmacopoeial Standards". This event was hosted by USP-EMA and EDQM, marking a confluence of experts in the establishment of reference standards from across the globe.
Speakers:
Stefan ALMELING, Head of Laboratory Department at EDQM
Christian ZEINE, Senior Scientific Affairs Manager at USP-EMA.
Our Representing Colleagues:
Svitlana CHYKALOVA
Tetiana YURCHENKO
Denis LEONTIEV
Elina KOTOVA
Viktoria KOVACH
Dmytro LEONTIEV
Natalia VOLOVYK.
The webinar fostered an environment of learning and collaboration. The discussion encompassed an array of topics, including the differences between pharmacopoeial reference standards and certified reference materials as well as their respective advantages and limitations, the identification of potential sources of variation and their contribution to measurement uncertainty, and the implementation of a risk-informed approach to the establishment of secondary reference standards.
Speakers underscored the possibility of using secondary reference standards in routine tests, emphasizing the prerequisite of demonstrating their suitability for intended use and traceability to primary reference standards, as well as the imperative to assess and account for their uncertainty in the overall measurement uncertainty.
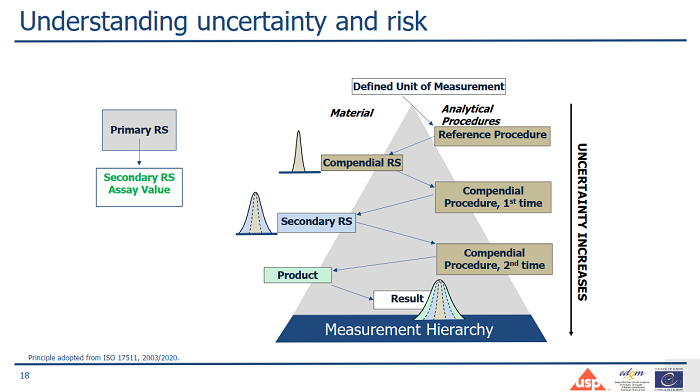
A snapshot showing the contribution of secondary reference standards to measurement uncertainty



