The report of the 57th meeting of the WHO Expert Committee on Pharmaceutical Specifications has been published.
New texts were approved and recommended for implementation, including:
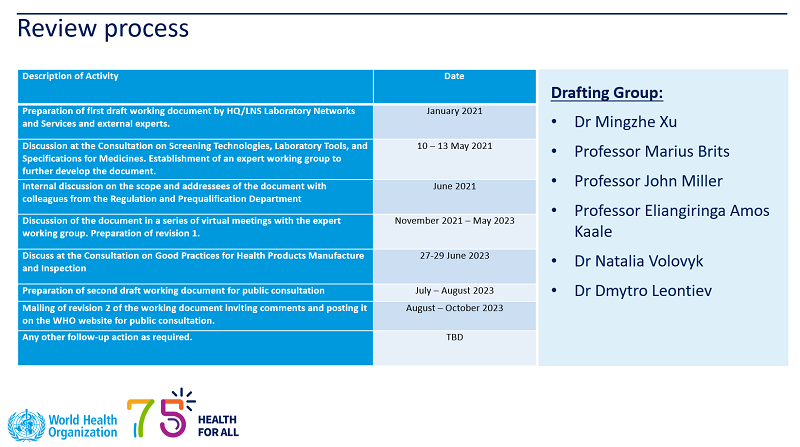
- WHO Good Practices for Pharmaceutical Quality Control Laboratories (also see WHO TRS 57, Annex 4),
in the revision of which Dmytro LEONTIEV and Natalia VOLOVYK, Deputy Director for Science and Deputy Director for Quality of the Ukrainian Scientific Pharmacopoeia Center for Quality of Medicines, respectively, took an active part.
The document introduces several new sections, among which Section 6.7 Measurement Uncertainty, which is based on the approach of the State Pharmacopoeia of Ukraine to the implementation of the concept of uncertainty in the pharmaceutical sector for compliance testing (§§ 6.63, 6.67, 6.68) and the following appendices:
- Target uncertainty and maximum permissible uncertainty for standard analytical operations for normal analytical practice (Appendix 2);
- The application of the concept of normal analytical practice for the evaluation/correction of estimated measurement uncertainty (Appendix 3).